Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 B
B ;
; 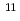 B NMR study
B NMR studyFujimoto, Tatsuya; Sakai, Hironori; Tokunaga, Yo; Kambe, Shinsaku; Walstedt, R. E.; Ikeda, Shugo; Matsuda, Tatsuma; Haga, Yoshinori; Onuki, Yoshichika
Physica B; Condensed Matter, 378-380, p.997 - 998, 2006/05
Times Cited Count:0 Percentile:0(Physics, Condensed Matter)We report 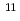 B NMR results of a single crystal UIr
B NMR results of a single crystal UIr B
B . The measurement was performed by Fast Fourier Transformation technique, because spectral line width is extremely narrow (
. The measurement was performed by Fast Fourier Transformation technique, because spectral line width is extremely narrow (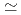 10 kHz) and NMR occurs in quite limited r.f. band due to a good sample quality. From temperature variation of Knight shift and nuclear spin-lattice relaxation rate, the development of ferromagnetic correlations was clarified in the temperature range above
10 kHz) and NMR occurs in quite limited r.f. band due to a good sample quality. From temperature variation of Knight shift and nuclear spin-lattice relaxation rate, the development of ferromagnetic correlations was clarified in the temperature range above 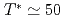 K. On the other hand, the magnetic dynamics was changed into another novel state below
K. On the other hand, the magnetic dynamics was changed into another novel state below 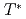 ; ferromagnetic correlations are restrained, meanwhile antiferromagnetic correlations are newly emerged. To explain this characteristic ground state, the one-dimensional
; ferromagnetic correlations are restrained, meanwhile antiferromagnetic correlations are newly emerged. To explain this characteristic ground state, the one-dimensional 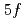 electrons state due to the unique structural lattice of UIr
electrons state due to the unique structural lattice of UIr B
B was proposed.
was proposed.
 (T=Fe, Co, Rh)
(T=Fe, Co, Rh)Maehira, Takahiro; Higuchi, Masahiko*; Hasegawa, Akira*
Physica B; Condensed Matter, 329-333(1-4), p.574 - 575, 2003/05
Times Cited Count:20 Percentile:66.95(Physics, Condensed Matter)The relativistic energy-band calculations have been carried out for 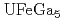 ,
, 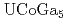 and
and 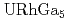 under the assumption that 5
under the assumption that 5 -electrons are itinerant. A hybridization between the U 5
-electrons are itinerant. A hybridization between the U 5 state and Ga 4
state and Ga 4 state occurs in the vicinity of the Fermi level. The Fermi surface of
state occurs in the vicinity of the Fermi level. The Fermi surface of 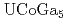 is quite similar to that of
is quite similar to that of 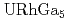 , which are all small in size and closed in topology.
, which are all small in size and closed in topology. 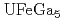 has the quasi-two-dimensional Fermi surface which looks like a lattice structure.
has the quasi-two-dimensional Fermi surface which looks like a lattice structure.
Tejima, Shogo
JNC TN8400 2000-029, 54 Pages, 2000/10
This report describes the study done by the author as a postdoctoral research associate at Japan Nuclear Cycle Development Institute. This report is divided into three parts: construction of a relativistic band calculation formalism based on the density functional theory, using this method, investigation of the electrical properties for ferromagnetic UGe and antiferromagnetic UO
and antiferromagnetic UO . (1)A relativistic band calculation (RBC) method. Band calculations for the s, p, and d electric structure have been developed well in the practical application and theoretical study. But band calculation method treating magnetic 5f electrons as actinide compounds are complicated and needed relativistic approach, so it is behind with the study of the 5f system. In this study we construct the relativistic band calculationformalism valid for magnetic 5f electrons. (2)Electric properties of UGe
. (1)A relativistic band calculation (RBC) method. Band calculations for the s, p, and d electric structure have been developed well in the practical application and theoretical study. But band calculation method treating magnetic 5f electrons as actinide compounds are complicated and needed relativistic approach, so it is behind with the study of the 5f system. In this study we construct the relativistic band calculationformalism valid for magnetic 5f electrons. (2)Electric properties of UGe . The actinide compounds UGe
. The actinide compounds UGe is ferromagnetic, so the theoretical analysis is not well yet. The electric structure and fermi surface of UGe
is ferromagnetic, so the theoretical analysis is not well yet. The electric structure and fermi surface of UGe are analyzed using the RBC. The theoretical results show that UGe
are analyzed using the RBC. The theoretical results show that UGe is heavy electron with the 5f character and are agreement with experimental one. (3)Electric structure of nuclear fuel UO
is heavy electron with the 5f character and are agreement with experimental one. (3)Electric structure of nuclear fuel UO . It is important to understand the mechanism of the thermal conductivity of nuclear fuel as antiferromagnetic UO
. It is important to understand the mechanism of the thermal conductivity of nuclear fuel as antiferromagnetic UO . The UO
. The UO band calculation reflecting the thermal properties, into account of relativistic effect, have not done yes. So using the RBC the detailed electric structure of UO
band calculation reflecting the thermal properties, into account of relativistic effect, have not done yes. So using the RBC the detailed electric structure of UO are obtained.
are obtained.

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO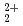 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Rai, D.*; Rao, L.*; Weger, H. T.*; GREGORY R.CHOPPI*; Yui, Mikazu
JNC TN8400 99-010, 95 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Pu(III), Am(III), and Cm(III) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(III) species are lacking, the data were selected based on chemical analogy to other trivalent actinides. In this study, the Pitzer ion-interaction model is mainly used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Rai, D.*; Rao, L.*; Weger, H. T.*; Felmy, A. R.*; Choppin, G. R.*; Yui, Mikazu
JNC TN8400 99-009, 115 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Th(IV), U(IV), Np(IV), and Pu(IV) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(IV) species was lacking, the data were selected based on chemical analogy to other tetravalent actinides. ln this study, the Pitzer ion-interaction model is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
; Nemoto, Takeshi; Numata, Koji; *; *; Hanawa, Eiji*; *
PNC TN8440 94-011, 19 Pages, 1994/04
None